Artificial Intelligence, Data Science & Machine Learning

Christian Poellabauer
Dr. Poellabauer is a full professor in the Knight Foundation School of Computer and Information Sciences and the Interim Associate Dean for Research and Graduate Studies in the College of Engineering and Computing at Florida International University.
He received his Ph.D. in Computer Science in 2004 and served on the faculty of the University of Notre Dame for 17 years before joining FIU in 2021.
At FIU, he leads the MOSAIC (Mobile Sensing and Analytics) Lab, which focuses on research topics such as digital health, wireless sensing, machine learning and artificial intelligence using time series data, the Internet-of-Things, and cybersecurity.
Most current research projects focus on the use of machine learning for health, e.g., leveraging data obtained from wireless sensors, wearable devices, or mobile devices to develop new tools for diagnosis and treatment of medical conditions such as neurodegenerative diseases, neurodevelopmental conditions, mental health problems, cardiovascular problems, etc.
His research has been funded by the NSF, NIH, Department of Defense, Department of Education, Toyota, Ford Research, the National Football League, National Geographic, GE Health, and IBM, among others. He was the recipient of an NSF CAREER Award in 2006 and named a Fulbright Scholar in 2018.
Project 1: Towards a Personalized Physical Intervention Recommender System for Parkinson’s Disease
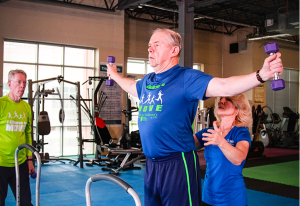
Summary: Neurodegenerative conditions, such as Parkinson’s Disease (PD), affect regions in the brain that play a key role in cognitive and motor function, leading to impairments of movement, speech production, memory, reasoning, planning, reflexes, among others. Assessment of disease state is essential in both clinical practice and research in order to measure disease severity, disease progression, and treatment effectiveness. Currently, the clinical assessment of PD relies primarily on subjective evaluations, such as rater-based interviews and questionnaires and tests such as simple rhythmic activities to evaluate motor symptoms and tremor. With the wealth of new mobile technologies, their sensor capabilities, and the resulting data, there are new opportunities to develop digital biomarkers that translate these new data sources into informative, actionable insights. This work utilizes advances in sensing, analytics, and machine learning to design integrated function-specific biomarkers that can provide fine-grained insights into a patient’s disease course for various neurological functions such as cognitive skills, fine and gross motor functions, speech/language, etc. This work also uses the newly developed biomarkers to investigate the effects of various intervention types on functional performance.
Project 2: Assessing Trauma Response of First Responders Using Physiological Biomarkers
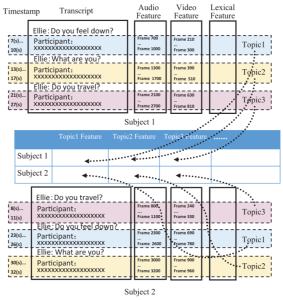
Summary: In the U.S. alone, over 20% of adults experienced a mental illness in 2019; a problem that has only worsened with the current pandemic. Mental health conditions, which include post-traumatic stress disorder, depression, suicidal ideation, and substance use disorder, are even more common among first responders. While first responders undergo extensive training, their demanding jobs often expose them to potentially traumatic events that can lead to short- and long-term mental health problems. This project will develop a continuous multi-modal mental health sensing and analysis framework able to detect subtle changes in psychological health using physiological sensor streams from wearable devices, specifically focusing on stress responses immediately after exposure to trauma, i.e., acute stress disorder (ASD) and acute post-traumatic stress disorder (PTSD). This approach will be able to detect and predict the occurrence of ASD and PTSD and provide an early alert mechanism that notifies first responder to seek help proactively.
Project 3: Assessing the Impact of Cancer Treatment on Autonomic Nervous System Function of Cancer Survivors
Summary:Dysautonomia is an umbrella term referring to a group of several medical conditions that cause a malfunction of the Autonomic Nervous System (ANS). Cancer can affect the ANS in a variety of ways and while cancer therapies have increased patient survival rates, their side effects (such as cardiotoxicity and neurotoxicity) can lead to autonomic nervous and cardiovascular system dysfunction. Autonomic dysfunction is an emerging, but also poorly understood topic. While it has been suggested as a risk factor for cardiovascular disease in breast cancer patients, there are gaps in the current knowledge and more research is needed to identify severity, predictors, and clinical correlates of cancer-related dysautonomia. Further, more research is needed to define beneficial interventions, e.g., exercise programs appear to promote physiological changes helping to reduce the decline of the autonomic modulation. This study aims to use a combination of frequent patient self-reports and continuous physiological measurements (based on wearable sensor technologies) to monitor dysautonomia in cancer patients and to analyze the effectiveness of various exercise programs.
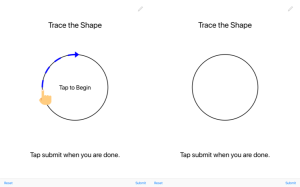
Project 4: Speech-Based Diagnosis of Neurological Trauma

Summary: Mild traumatic brain injuries (mTBI) such as concussions are arguably one of the most pressing concerns in sports today, with an estimated 2-4 million cases every year in youth sports alone. The potential short- and long-term impacts on the health and well-being of individuals with brain injuries are extensive. For example, individuals with mTBI may display a range of somatic, affective, and cognitive symptoms such as headaches, depression, loss of memory, and loss of brain function (collectively known as Post Concussion Syndrome). The effects of untreated concussions, e.g., leading to diseases such as chronic traumatic encephalopathy (which has been tied to depression and suicide), an elevated incidence of Alzheimer Disease (and a reduced age of onset for Alzheimers), and dementia developing at a higher rate and a younger age. It is therefore imperative to accurately detect and appropriately treat mTBI, especially among adolescents, whose brains are still developing and more prone to long-term damage. This research aims to identify a novel biomarker that can only be extracted and utilized using modern computing and speech analysis technology. Prior research has provided evidence of a link between neurological functioning and acoustic features of the human voice, but these links are so subtle that they have been difficult to capture. Our findings will provide the foundation for a new generation of mTBI assessment tools utilizing advances in mobile technology and speech processing.